1 mole hydrogen|1 mole hydrogen to grams : Clark The molar mass of a substance is the mass in grams of 1 mole of the substance. As shown in this video, we can obtain a substance's molar mass by summing the molar masses of its component atoms. We can then use the calculated molar mass to . Aprenda a tocar a cifra de Azul da Cor do Mar (Tim Maia) no .
0 · weight of 1 mole hydrogen
1 · moles of hydrogen calculator
2 · molar mass for hydrogen
3 · mass of 1 mole hydrogen
4 · liquid hydrogen molar mass
5 · hydrogen moles to weight
6 · hydrogen mass calculator
7 · More
8 · 1 mole hydrogen to grams
WEBComo ver Ted Lasso? Descubra todas as ofertas de streaming disponíveis para assistir .
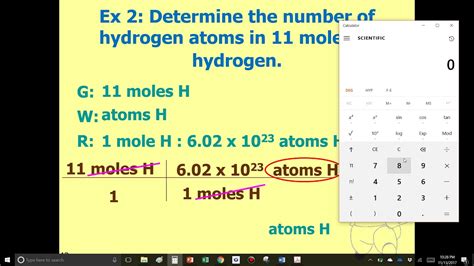
1 mole hydrogen*******Using the concept of the mole, we can now restate Dalton’s theory: 1 mol of a compound is formed by combining elements in amounts whose mole ratios are small whole numbers. For example, 1 mol of water (H 2 O) has 2 mol of hydrogen atoms and 1 mol of . One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). The number 6.022 × 10²³ is known as Avogadro's number or Avogadro's . Well, one mole of hydrogen ATOMS has a mass of #1*g#. Explanation: And one mole of dihydrogen molecules have a mass of #2*g# .the atomic masses are .
The molar mass of a substance is the mass in grams of 1 mole of the substance. As shown in this video, we can obtain a substance's molar mass by summing the molar masses of its component atoms. We can then use the calculated molar mass to .
There are 4 easy steps to find the molar mass of H based on its chemical formula. 1. Count The Number of Each Atom. The first step to finding the molar mass of Hydrogen is to .Balanced chemical equations are balanced not only at the molecular level, but also in terms of molar amounts of reactants and products. Thus, we can read this reaction as “two .Molar mass is the mass (in atomic mass units) of one mole of a of a substance. One atomic mass unit (u) is equal to 1/12 the mass of one atom of carbon-12. It is also sometimes .
The mass of 1 mol of a substance is referred to as its molar mass, whether the substance is an element, an ionic compound, or a covalent compound. Example .The Molar mass or Molecular Weight (interchangeable terms so long as we are on Earth) of a substance is the total of all the individual masses of the elements it contains. To use our old friend water as an example: One .
Under ordinary conditions, hydrogen gas is a loose aggregation of hydrogen molecules, each consisting of a pair of atoms, a diatomic molecule, H 2. The .Volume of one mole of gas at standard temperature and pressure, stp, (0 °C, 101,500 N m –2) is 22.4 dm 3. At room temperature and average pressure, rtp, the students can expect an answer of approximately 24 . This molecule and its molecular formula indicate that per mole of methane there is 1 mole of carbon and 4 moles of hydrogen. In this case, the mole is used as a common unit that can be applied to a ratio as shown below: \[2 \text{ mol H } + 1 \text{ mol O }= 1 \text{ mol } \ce{H2O} \nonumber\] In this this chemical reactions, the moles of H . The molar mass is the mass of a mole of a substance, which corresponds to the quantity of matter that contains an Avogadro's number of atoms or molecules (depending on the substance). Molar masses change widely in the periodic table: a mole of hydrogen weighs only 1.008 g, while a mole of uranium weighs 238.03 g. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms, molecules, or other specified particles. . The atomic weight of one atom of hydrogen is 1.008 amu, so that of two atoms is 2.016. The atomic weight of one atom of oxygen is 15.999, so the molar mass of water is 2.016 .
Hydrogen is a chemical element; it has symbol H and atomic number 1. It is the lightest element and, at standard conditions, is a gas of diatomic molecules with the formula H2, sometimes called dihydrogen, [11] but more commonly called hydrogen gas, molecular hydrogen or simply hydrogen. It is colorless, odorless, tasteless, [12] non-toxic, and .Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in H: Molar Mass (g/mol) H (Hydrogen) 1 × 1.00794 = 1.00794. 4. Sum Each Element's Mass. Finally, add together the total mass of each element to get the molar mass of H:

One mole of glycine, C 2 H 5 O 2 N, contains 2 moles of carbon, 5 moles of hydrogen, 2 moles of oxygen, and 1 mole of nitrogen: The provided mass of glycine (~28 g) is a bit more than one-third the molar mass (~75 g/mol), so the computed result is expected to be a bit greater than one-third of a mole (~0.33 mol). Dividing the compound’s mass .Here are some examples. The mass of a hydrogen atom is 1.0079 u; the mass of 1 mol of hydrogen atoms is 1.0079 g. Elemental hydrogen exists as a diatomic molecule, H 2. One molecule has a mass of 1.0079 + 1.0079 = 2.0158 u, while 1 mol H 2 has a mass of 2.0158 g. A molecule of H 2 O has a mass of about 18.01 u; 1 mol H 2 O has a mass of 18.01 g .
The mole concept can be extended to masses of formula units and molecules as well. The mass of 1 mol of molecules (or formula units) in grams is numerically equivalent to the mass of one molecule (or formula unit) in atomic mass units. For example, a single molecule of O 2 has a mass of 32.00 u, and 1 mol of O 2 .
About Hydrogen peroxide; Hydrogen peroxide weighs 1.4425 gram per cubic centimeter or 1 442.5 kilogram per cubic meter, i.e. density of hydrogen peroxide is equal to 1 442.5 kg/m³; at 25°C (77°F or 298.15K) at standard atmospheric pressure.In Imperial or US customary measurement system, the density is equal to 90.05233 pound per cubic foot .One mole of any substance has a mass equal to its atomic or molecular weight in grams. For example, one mole of carbon-12 atoms weighs 12 grams, while one mole of water (H2O) molecules weighs 18 grams (2 hydrogen atoms weigh 1 gram each, and 1 oxygen atom weighs 16 grams). This relationship between moles and mass is known as the .
The mole is simply a very large number, #6.022 xx 10^23#, that has a special property.If I have #6.022 xx 10^23# hydrogen atoms, I have a mass of 1 gram of hydrogen atoms.If I have #6.022 xx 10^23# #H_2# molecules, I have a mass of 2 gram of hydrogen molecules. If I have #6.022 xx 10^23# #C# atoms, I have (approximately!) 12 grams.. The mole is .
1 mole hydrogen 1 mole hydrogen to grams And thus, "mass of 1 mole of hydrogen atoms" = 1.6727xx10^(-27)*"kg"xx6.0225xx10^23*mol^-1xx10^(3)*"g"*kg^-1 =1.007*"g"*"mol"^-1 Clearly, you would not be expected to know these masses. You would be expected to be able to do such a calculation if provided with the masses of the nucleon, and with "Avogadro's number". .Calculating the mass of 1 mole of hydrogen(H): Step 1: Analyzing the given data. Number of moles of H = 1 mol. Molecular weight of H = 1 gmol-1. Step 2: Calculating the mass of 1 mole of hydrogen. Formula for calculating the number of moles. n = m M where, n = number of moles m = given mass M = molecular mass. Put the value. 1 mol = m 1 g .
1 mole hydrogen to gramsOne mole of any substance has a mass equal to its atomic or molecular weight in grams. For example, one mole of carbon-12 atoms weighs 12 grams, while one mole of water (H2O) molecules weighs 18 grams (2 hydrogen atoms weigh 1 gram each, and 1 oxygen atom weighs 16 grams). This relationship between moles and mass is known as the .The mole is simply a very large number, #6.022 xx 10^23#, that has a special property.If I have #6.022 xx 10^23# hydrogen atoms, I have a mass of 1 gram of hydrogen atoms.If I have #6.022 xx 10^23# #H_2# molecules, I have a mass of 2 gram of hydrogen molecules. If I have #6.022 xx 10^23# #C# atoms, I have (approximately!) 12 grams.. The mole is . And thus, "mass of 1 mole of hydrogen atoms" = 1.6727xx10^(-27)*"kg"xx6.0225xx10^23*mol^-1xx10^(3)*"g"*kg^-1 =1.007*"g"*"mol"^-1 Clearly, you would not be expected to know these masses. You would be expected to be able to do such a calculation if provided with the masses of the nucleon, and with "Avogadro's number". .
Calculating the mass of 1 mole of hydrogen(H): Step 1: Analyzing the given data. Number of moles of H = 1 mol. Molecular weight of H = 1 gmol-1. Step 2: Calculating the mass of 1 mole of hydrogen. Formula for calculating the number of moles. n = m M where, n = number of moles m = given mass M = molecular mass. Put the value. 1 mol = m 1 g . 1 Answer. The molecular formula of methane ( CH 4) suggests that one molecule of this compound consists of 4 atoms of H. So 1 mole of CH 4 will have 4 mol atom of Hydrogen. Now number of moles of CH 4 in 5.2 × 1024 molecules is. = 5.2 × 1024 Avogadro's number. So the number of moles of H atom in it. = 4 ×5.2 × 1024 Avogadro's .The mass of a hydrogen atom is 1.0079 amu; the mass of 1 mol of hydrogen atoms is 1.0079 g. Elemental hydrogen exists as a diatomic molecule, H 2. One molecule has a mass of 1.0079 + 1.0079 = 2.0158 amu, while 1 mol H 2 has a mass of 2.0158 g. A molecule of H 2 O has a mass of about 18.01 amu; 1 mol H 2 O has a mass of 18.01 g.
The most useful quantity for counting particles is the mole. So if each coefficient is multiplied by a mole, the balanced chemical equation tells us that 1 mole of nitrogen reacts with 3 moles of hydrogen to produce 2 moles of ammonia. This is the conventional way to interpret any balanced chemical equation.
A mole (mol) is a number of things equal to the number of atoms in exactly 12 g of carbon-12. Experimental measurements have determined that this number is very large: 1 mol = 6.02214179 × 10 23 things. Understand that a mole means a number of things, just like a dozen means a certain number of things-twelve, in the case of a dozen.1 mole hydrogen The mole is a key unit in chemistry. The molar mass of a substance, in grams, is numerically equal to one atom's or molecule's mass in atomic mass units. 7.1: The Mole and Avogadro’s Number is shared under a CC BY-NC-SA 3.0 license and was authored, remixed, and/or curated by LibreTexts. A mole is \ (6.022×10^ {23}\) things.4. Sum Each Element's Mass. Finally, add together the total mass of each element to get the molar mass of H2: 2.01588 g/mol = 2.01588 g/mol. 5. Find Mole Fraction. To find the mole fraction and percentage of each element in H2, divide each total from step 3 by the total molar mass found in step 4: Mole Fraction.1 amu = (1gram)/(6.022*10 23) = 1.66*10-24 grams. Therefore, the mass of one mole of an element will be equal to its atomic mass in grams. Number of Electrons in a Mole of Hydrogen Molecule. The number of electrons in a mole of hydrogen molecule is. 1 mole of H 2 contains 6.023×10 23 molecules and each molecule of H 2 contains two .How many grams Hydrogen in 1 mol? The answer is 1.00794. We assume you are converting between grams Hydrogen and mole. You can view more details on each measurement unit: molecular weight of Hydrogen or mol The molecular formula for Hydrogen is H. The SI base unit for amount of substance is the mole. 1 grams .
Làm thế nào để tạo tài khoản tại Live Casino House? 1. Tài khoản Email (Sử dụng email thật) 2. Mật khẩu (Tối thiểu 8 ký tự đăng ký và bao gồm 1 con số trong mật khẩu) 3. Số .
1 mole hydrogen|1 mole hydrogen to grams